s22- lewis structure|hydrogen ion lewis structure : Clark This video gives detailed information on Lewis structure of Sulphur & Sulphide ion. In Lewis dot structure, valence electrons are represented in the form of dots. WEBIn theatres 30 June 2023 brought to you by Disney. Directed by: James Mangold. Starring: Harrison Ford, Phoebe Waller-Bridge, Antonio Banderas, John Rhys-Davies, Shaunette Renee Wilson, Thomas Kretschmann, Toby Jones, Boyd Holbrook, Oliver Richters, Ethann Isidore, Mads Mikkelsen.
0 · sulphur lewis dot structure
1 · sulfur lewis dot structure
2 · s2 lewis structure
3 · s2 lewis dot diagram
4 · lewis dot s2 structure
5 · lewis diagram calculator
6 · hydrogen ion lewis structure
7 · electron dot structure for sulfur
8 · More
Agenda das temporadas inéditas. Descubra os 7 episódios da 1ª temporada da série I'm A Virgo.
s22- lewis structure*******This video gives detailed information on Lewis structure of Sulphur & Sulphide ion. In Lewis dot structure, valence electrons are represented in the form of dots.The Lewis electron structure is drawn within brackets as is customary for an ion, with the overall charge indicated outside the brackets, and the bonding pair of electrons is .The dot structure for sulfur dioxide has sulfur with a double bond to an oxygen on the left, and two lone pairs of electrons on that oxygen, and the sulfur with a double bond to an .hydrogen ion lewis structureLewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or . The Lewis structure of S2- is represented by the capital letter “S” that is surrounded by eight dots, including a “2-” superscript that indicates the charge of the . SO2 Lewis Structure. Before directly jumping into the lewis structure of SO2, let’s have a quick discussion regarding the importance of lewis structure and the steps to draw it. Lewis structure is the . Introduction to Lewis structures. A Lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. The .Lewis Structures. We also use Lewis symbols to indicate the formation of covalent bonds, which are shown in Lewis structures, drawings that describe the bonding in molecules and polyatomic ions. For example, .
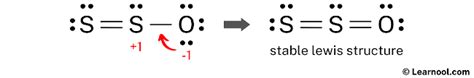
SO2 Molecular Geometry and Shape. SO2 Valence Electrons. To form the Lewis structure of Sulfur Dioxide, we need first to determine the number of valence electrons available. These valence .Your solution’s ready to go! Our expert help has broken down your problem into an easy-to-learn solution you can count on. Question: Draw the Lewis structure for the polyatomic trisulfide (S ) anion. Be sure to include all .3. 2-. (Thiosulfate) Lewis Structure. Thiosulfate ion contains two sulfur atoms and three oxygen atoms. In lewis structure of S 2 O 32- ion, there is -2 charge and oxygen atoms should hold them. Total valence electrons .s22- lewis structureYou'll get a detailed solution from a subject matter expert that helps you learn core concepts. See Answer. Question: Be sure to answer all parts. Draw Lewis structures for the following ions. Be sure to include all lone pair electrons and nonzero formal charges. S22−. Be sure to answer all parts. Draw Lewis structures for the following ions.s22- lewis structure hydrogen ion lewis structureHere’s the best way to solve it. QUESTIONS Which of the following represent the Lewis structure for S2-? A):S:2- B) 32- C):S:- D) $:2- E) S:2- choice A choice choice choice D choice QUESTION O Give the condensed electron configuration for iron (Fo). Use the drop-down menu to make your selections.
The protons and electrons of an atom contain positive and negative charges of +1 and -1 respectively. If the total numbers of these sub-atomic particles are balanced, then the atom itself is electrically neutral. If there is an excess of one or more electrons, then a negatively charged simple anion is formed.The structure on the right is the Lewis electron structure, or Lewis structure, for H 2 O. With two bonding pairs and two lone pairs, the oxygen atom has now completed its octet. Moreover, by sharing a bonding pair with oxygen, each hydrogen atom now has a full valence shell of two electrons. Chemists usually indicate a bonding pair by a single .
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a “skeleton structure.”. Lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Lewis electron dot diagrams for ions have less (for cations) or more (for anions) dots than the corresponding atom. This page titled 9.2: Lewis Electron Dot Diagrams is shared under a CC BY-NC-SA 3.0 license and was authored, remixed, .We use Lewis symbols to describe valence electron configurations of atoms and monatomic ions. A Lewis symbol consists of an elemental symbol surrounded by one dot for each of its valence electrons: Figure 7.9 shows the Lewis symbols for the elements of the third period of the periodic table. Figure 7.9 Lewis symbols illustrating the number of . Follow these simple steps to draw Lewis dot structures: Draw the atoms on paper and put dots around them to represent valence electrons of the atom. Be sure to have the correct number of electrons. If the species is an ion, add or subtract electrons corresponding to the charge of the ion. Add an electron for every negative (-) charge, .The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is usually used to indicate a shared pair of electrons: In the Lewis model, a single shared pair of electrons is a single bond. DO NOT FORGET TO SUBSCRIBE!LinkedIn: https://www.linkedin.com/in/kevan-j-english-91b9b11b7/Snapchat: https://www.snapchat.com/add/kravonoInstagram: https://w. Step 2: Select the central atom. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now here the given molecule is SiS2 (silicon disulfide) and it contains silicon atom (Si) and sulfur atoms (S). You can see the electronegativity values of silicon atom (Si) and sulfur atom (S . The Lewis structure of S2- is represented by the capital letter “S” that is surrounded by eight dots, including a “2-” superscript that indicates the charge of the ion. The dots denote the total valence electrons in the ion’s outermost shell. A Lewis structure, also referred to as a Lewis electron dot diagram, is a graphic depiction .The Lewis structure indicates that each Cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between the atoms). A dash (or line) is usually used to indicate a shared pair of electrons: In the Lewis model, a single shared pair of electrons is a single bond. DO NOT FORGET TO SUBSCRIBE!LinkedIn: https://www.linkedin.com/in/kevan-j-english-91b9b11b7/Snapchat: https://www.snapchat.com/add/kravonoInstagram: https://w.
Step 2: Select the central atom. For selecting the center atom, you have to remember that the atom which is less electronegative remains at the center. Now here the given molecule is SiS2 (silicon disulfide) and it contains silicon atom (Si) and sulfur atoms (S). You can see the electronegativity values of silicon atom (Si) and sulfur atom (S .

The Lewis structure of S2- is represented by the capital letter “S” that is surrounded by eight dots, including a “2-” superscript that indicates the charge of the ion. The dots denote the total valence electrons in the ion’s outermost shell. A Lewis structure, also referred to as a Lewis electron dot diagram, is a graphic depiction .
Organic Chemistry Lewis Structures and Bonding Lewis Dot Diagram. 1 Answer Sam Jun 10, 2016 . Explanation: Sulfur belongs to group 16. It has 6 valence electrons. A #\ 2- # charge indicates that the atom has gained 2 electrons. This makes the total number of valence electrons equal to 8.Draw the Lewis structure for the polyatomic trisulfide (S 3 2-) anion. Be sure to include all resonance structures that satisfy the octet rule. There are 2 steps to solve this one. Step 1. S contain 6 valence electrons. In S 3 2-, there are -2 charge which means two S-atom have -1 charge o. View the full answer. Step 2. Unlock.Complete the Lewis structures of these molecules by adding multiple bonds and lone pairs. Do not add any more atoms. (a) the amino acid serine: (b) urea: (c) pyruvic acid: (d) uracil: (e) carbonic acid: A compound with a molar mass of about 28 g/mol contains 85.7% carbon and 14.3% hydrogen by mass. Write the Lewis structure for a molecule of .
The Lewis electron structure for the NH 4+ ion is as follows: The nitrogen atom shares four bonding pairs of electrons, and a neutral nitrogen atom has five valence electrons. Using Equation 4.4.1, the formal charge on the nitrogen atom is .
It mostly has two types of dots ×&∘ × & ∘ that represent electrons from two different atoms. Complete Step By Step Answer: The inorganic anion of sulphur atoms is known as sulphide. It has the chemical formula S2− S 2 − . The sulphide can have one or more S2− S 2 − anions. Large families of inorganic and organic compounds like . A step-by-step explanation of how to draw the Lewis dot structure for Sr (Strontium). I show you where Strontium is on the periodic table and how to determi. Figure 2.4.2 demonstrates the use of Lewis symbols to show the transfer of electrons during the formation of ionic compounds. Figure 2.4.2: Cations are formed when atoms lose electrons, represented by fewer Lewis dots, whereas anions are formed by atoms gaining electrons. The total number of electrons does not change. Step 3: Connect each atoms by putting an electron pair between them. Now in the SeS2 molecule, you have to put the electron pairs between the selenium atom (Se) and sulfur atoms (S). This indicates that the selenium (Se) and sulfur (S) are chemically bonded with each other in a SeS2 molecule. Step 4: Make the outer atoms stable.
Resultado da Defeat all 9 bosses in Amirdrassil, The Dream's Hope raid with our .
s22- lewis structure|hydrogen ion lewis structure